|
Dynamic Chiropractic June 2018
Pain is a huge problem in America, and all Americans know that opiate drugs are not the solution to our pain problem. In her 2014 book, A Nation in Pain, Judy Foreman claims, “Out of 238 million American adults, 100 million live in chronic pain.” (1) A conservative estimate of the direct costs and lost productivity resulting from this pain is up to $635 billion yearly (2). Chronic pain affects every region of the body. Quantifying the anatomical regions for American’s chronic pain shows that the most significantly affected region is the lower back followed by (3):
Lower-Back Pain 28.1%
Knee Pain 19.5%
Severe Headache 16.1%
Neck Pain 15.1%
Shoulder Pain 09.0%
Finger Pain 07.6%
Hip Pain 07.1%
There is a high level of satisfaction by patients who seek treatment for spinal and other pain syndromes from chiropractors (4). It is argued that the physiological basis for the chiropractic benefit for pain suffers is the closure of the “pain gate.” (5) Yet, chiropractors often also use adjuncts to spinal adjusting for the treatment of pain syndromes. The rational for such adjuncts is that inflammation alters the threshold of the nociceptive system.
Most pain is a chemical event. Inflammatory chemicals irritate pain nerves. Understanding of the inflammatory chemical nature of pain was awarded the Nobel Prize in Physiology or Medicine in 1982. In 2007, the journal Medical Hypothesis states (6):
“Every pain syndrome has an inflammatory profile consisting of the inflammatory mediator that are present in the pain syndrome. “The key to treatment of Pain Syndromes is an understanding of their inflammatory profile.” “The origin of all pain is inflammation and the inflammatory response.”
“Irrespective of the type of pain whether it is acute or chronic pain, peripheral or central pain, nociceptive or neuropathic pain, the underlying origin is inflammation and the inflammatory response.”
“Irrespective of the characteristic of the pain, whether it is sharp, dull, aching, burning, stabbing, numbing or tingling, all pain arises from inflammation and the inflammatory response.
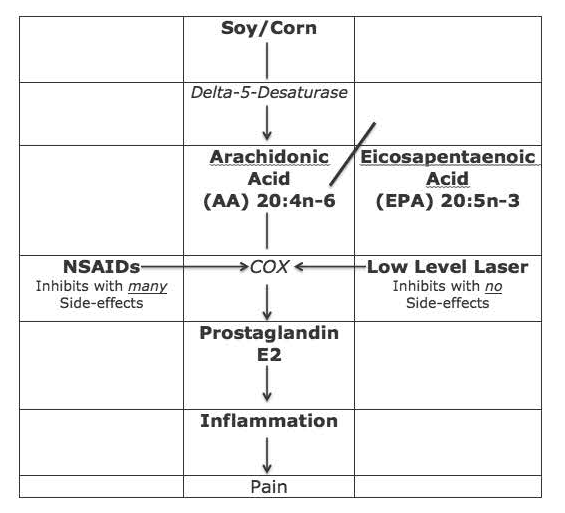
Although there are many inflammatory chemicals related to pain, the best understood is an eicosanoid hormone-like chemical called Prostaglandin E2 (PGE2). As depicted in the graph above, PGE2 is derived from the omega-6 fatty acid Arachidonic Acid (AA). AA itself is derived from vegetable oils, primarily corn, soy, sunflower, safflower, cottonseed, peanut, and canola oils. As such, consumption of any of these vegetable oils is inflammatory and related to pain thresholds. When one consumes meat derived from animals fed any of these products one is consuming high quantities of pre-formed AA, increasing the likelihood of suffering from pain syndromes (7).
In the pain references text, Weiner’s Pain Management, published by the American Academy of Pain Management, it notes the following as a major cause of the modern epidemic of pain (8):
“Changes in the modern diet are largely responsible for the increasing incidence of essential fatty acid imbalances and deficiencies.”
“The ratio of omega-6 to omega-3 fats has changed dramatically due to the widespread use of vegetable oils (mostly n-6 fats) in cooking and to the processing of oils.”
“Historical estimates place the ratio of omega-6 to omega-3 oils at nearly 1:1 for prehistoric humans.”
By the turn of last century (1900), the ratio had increased to about 4:1. The current American ratio is about 25:1.
This text indicates that 100 years ago Americans consumed about two pounds of these inflammatory vegetable oils yearly; the current approximate level of consumption in 25 pounds yearly. The results are an explosion of inflammatory conditions, including pain, which are treated with non-steroidal anti-inflammatory drugs (NSAIDs).
As noted in the graph below on left, the conversion of the omega-6 fatty acid AA into inflammatory (and pain producing) PGE2 is controlled by a series of enzymes called cyclooxygenase, often abbreviated COX. The 1982 Nobel Prize details how the COX enzymes are inhibited by a group of drugs, mentioned in Weiner’s text, non-steroidal anti-inflammatory drugs (NSAIDs). However, when these drugs (NSAIDs) are taken for chronic pain, there are many serious side effects. In 2003, the journal Spine states (9):
“Adverse reactions to non-steroidal anti-inflammatory (NSAID) medication have been well documented.”
“Gastrointestinal toxicity induced by NSAIDs is one of the most common serious adverse drug events in the industrialized world.”
In 2006, the journal Surgical Neurology states (10): Blockage of the COX enzyme inhibits the conversion of arachidonic acid to the very pro-inflammatory prostaglandins that mediate the classic inflammatory response of pain.
Almost all patients who take the long-term NSAIDs will have gastric hemorrhage, 50% will have dyspepsia, 8% to 20% will have gastric ulceration, 3% of patients develop serious gastrointestinal side effects, which results in more than 100,000 hospitalizations, an estimated 16,500 deaths, and an annual cost to treat the complications that exceeds 1.5 billion dollars.
“NSAIDs are the most common cause of drug-related morbidity and mortality reported to the FDA and other regulatory agencies around the world.”
Other side effects attributed to the consumption of NSAIDs includes end-stage renal disease, heart attacks, strokes, erectile dysfunction, hearing loss, deep vein thrombosis, and increased risk of Alzheimer’s disease
A modern and proven effective non-drug approach to the treatment of pain, especially chronic pain, is the supplementation of omega-3 fatty acids, especially eicosapentaenoic acid (EPA), found in fish oil. EPA is a precursor to the anti-inflammatory eicosanoid Prostaglandin E3 (PGE3). The only drawback to this approach (supplementation with high-dose quality fish oil) is the optimum benefit takes months of ingestion (10, 11, 12).
Is there a way to inhibit the COX enzymes for the treatment of both acute and chronic pain that is quick, effective, and safe, without side effects? The answer is yes, the application on low-level laser therapy.
A search of the National Library of Medicine using PubMed (3/29/2018) with the search terms “low-level laser therapy AND pain” locates 1,161 citations. A search of the National Library of Medicine using PubMed (3/29/2018) with the search terms “low-level laser therapy AND NSAID” locates 66 citations. Laser Therapy reference texts emphasize the ability of low-level laser to effectively treat pain without side effects. For example, the 2010 book titled The New Laser Therapy Handbook states (13):
“The effect of laser photon therapy [LPT] on inflammation is covered in many chapters of this book. The anti-inflammatory effect of LPT has been widely studied. LPT seems to have a similar effect to steroids and NSAIDs, but without the severe side effects of these very common pharmaceuticals.”
The authors indicate that laser therapy for pain control is enhanced if the laser is applied to both the painful region and the nerve roots that innervate the painful region. Other studies further emphasize the importance of applying the laser to the nerve roots. A particularly interesting study resulted in the successful management of 35 patients suffering from carpal tunnel syndrome; the laser therapy was not applied to the wrist but rather to the nerve roots that innervate the wrist (14).
There are advantages to being capable of doing both regions simultaneously.
An important study documenting the ability of low-level laser therapy to inhibit COX enzymes resulting in a significant reduction in inflammatory chemicals was published not in a laser journal, but rather in a cancer journal, Support Care Cancer (15). After exposing animals to caustic inflammatory chemicals (fluorouracil), they were randomly assigned to either a control group (no laser) or to low-level laser therapy. At four time points, tissue samples were immunohistochemistry assessed from both groups by a blinded examiner. The authors found that the laser therapy group “was accompanied by a significantly lower level of COX-2 staining.”
Interestingly, increasing the laser fluence (increasing the milliwatts by 3X) did not further enhance the clinical outcomes. In contrast, such higher laser fluence reduced the clinical benefit to the level of the control group (no laser). This suggests that laser therapy is not linear, but rather hormetic (bell shaped curve). The authors concluded:
“The tissue response to laser therapy appears to vary by dose. Low-intensity laser therapy appears to reduce the severity of mucositis, at least in part, by reducing COX-2 levels and associated inhibition of the inflammatory response.”
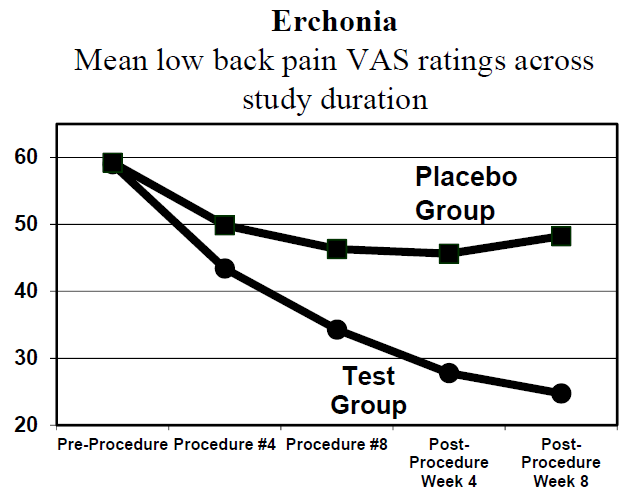
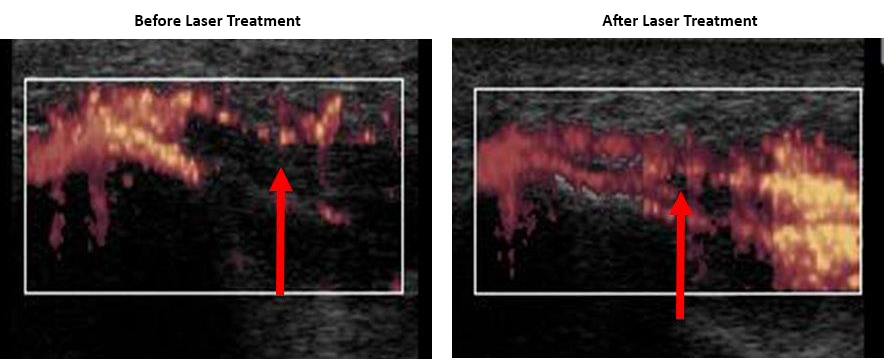
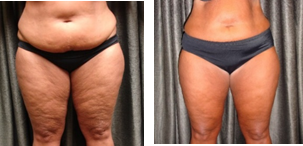
Thousands of chiropractors are using low-level laser therapy as an adjunct to chiropractic and nutrition in the management of their pain patients. Low-level laser therapy is quick, effective, and safe, and earned FDA clearance in 2002. (Erchonia Corporation www.Erchonia.com)
|