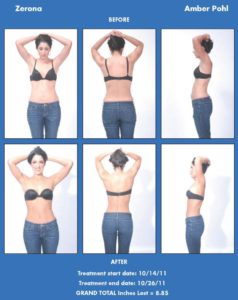
Model Wins a Trip to Florida, $5,000 cash and Stars in Zerona’s 2012 Marketing Campaig
Erchonia, the global leader in low level laser healthcare applications, has launched a “Zerona Model Search” contest to find the next face—and body—of Erchonia’s 2012 marketing campaign for the FDA-approved Zerona non-invasive, painless, body contouring laser.
Erchonia is searching for someone who used the Zerona procedure to reduce inches from his or her waist, hips and thighs and would be interested in sharing the experience with others. Erchonia will award the winner a cash prize of $5,000, plus a four-night vacation to Miami, Florida, including airfare and hotel stay for two. The winner will also be photographed in a professional photo shoot and will be featured in Erchonia’s marketing campaign for one year.
To enter, visit Zerona’s Facebook page (www.Facebook.com/myzerona) to submit your name, address, phone number, a photo of you both before and after your Zerona procedure, what practice treated you, how many inches you lost with Zerona, and why you deserve to win. Submissions must be received by December 31, 2011.
“We’ve been so inspired by stories we’ve heard about how the Zerona procedure has really changed people’s lives, we thought this contest would be a fun way to learn more and reward people for accomplishing their goals,” says Charlie Shanks, vice president of Erchonia.
“Although we are only looking for someone who actually went through the Zerona procedure, the winner might not necessarily be the person who lost the most inches total. We are really interested in finding someone with a compelling story who encompasses the excitement, substance and sophistication of the brand,” adds Shanks.
Using low level laser light, the Zerona laser creates a pore in fat cells and stimulates fat to leak out of the cell. The cell deflates, but is not destroyed, and the fat is naturally processed by the lymphatic system as waste. After six treatments, one every other day for two weeks, patients lose an average of 3.65 inches from their waists, hips and thighs without pain, side effects or downtime. Zerona was approved by the FDA for non-invasive circumferential reduction based on a recent double-blind, placebo-controlled, multi-site clinical trial.
For more information, please visit www.myzerona.com. Follow Zerona on Twitter at www.Twitter.com/myzeronaand on Facebook, www.Facebook.com/myzerona.
About Erchonia
Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years Erchonia has been conducting research and development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven safe and effective through independent clinical trials. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, accelerate healing, and treat acne. For additional information, visitwww.erchonia.com.
Susan Woods, Crier Communications, 310-274-1072 x 202 susan@crierpr.com
 Depending on the settings selected, the
Depending on the settings selected, the