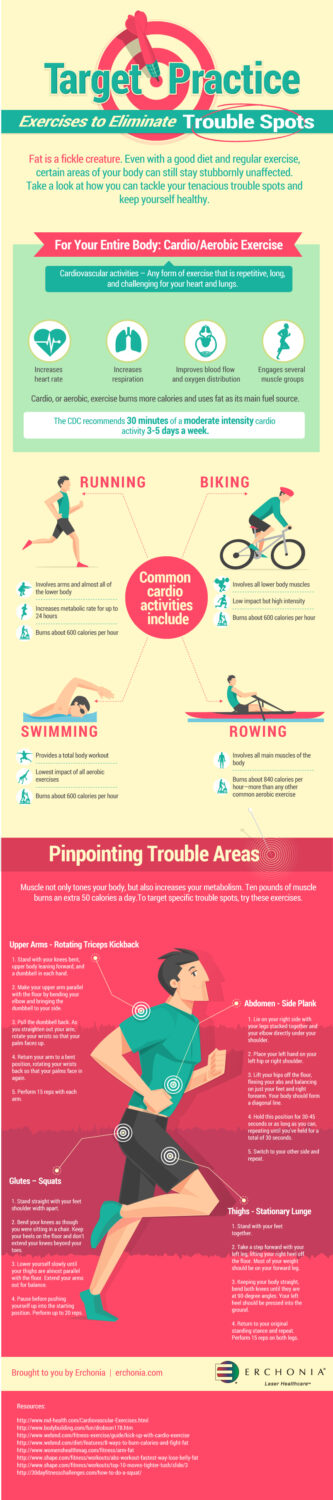
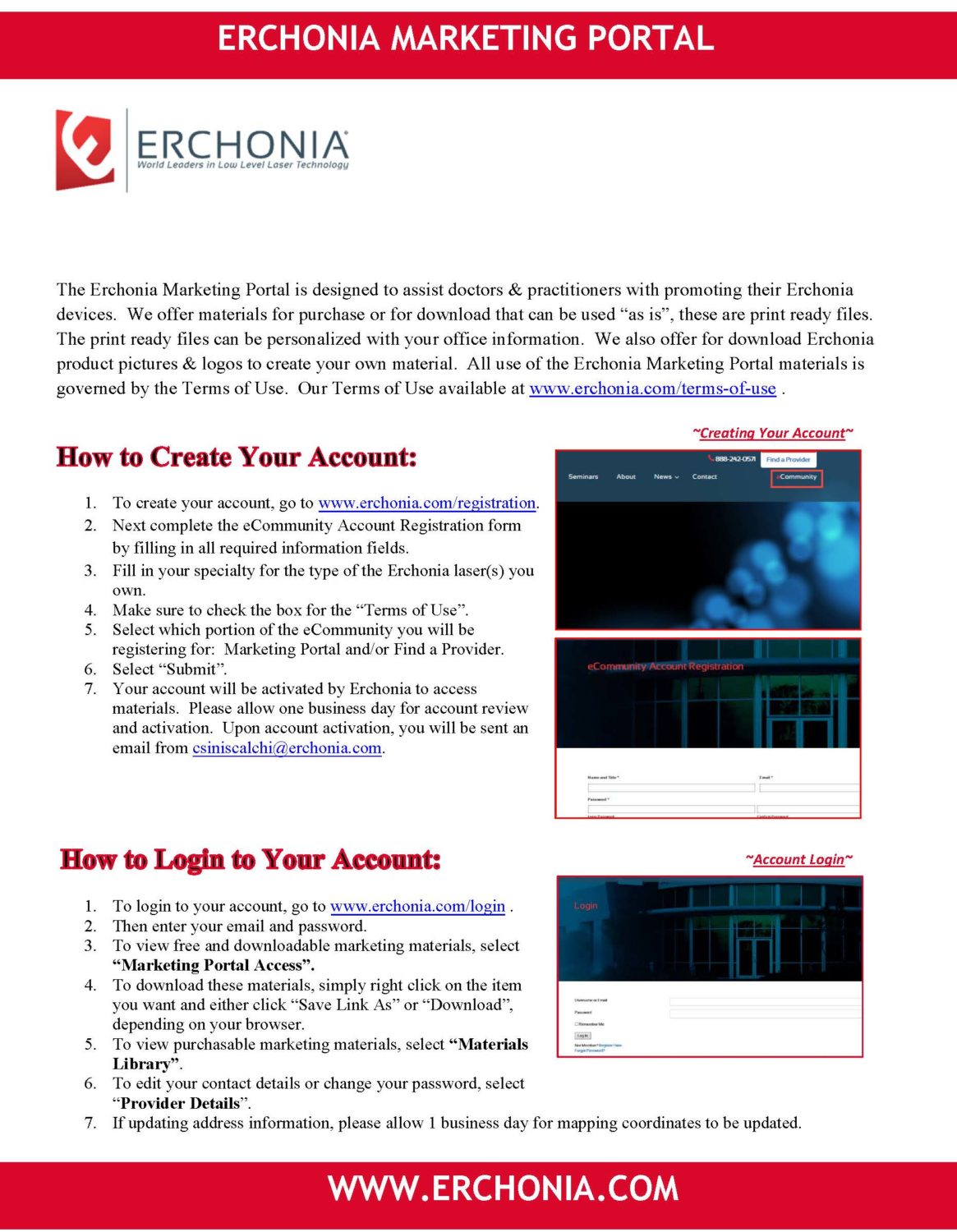
People are under the common misconception that staying healthy means eating less. Far from causing fat loss, skipping meals actually causes numerous health problems, including:
- A slowed-down metabolism
- Deteriorating muscles
- General fatigue
- Impaired concentration
Contrary to what you think, eating can help you lose weight. It’s just a matter of finding the right foods to eat. Let’s take a look at some foods that can help you shed pounds and improve your health.
- Eggs
Doctors, dieticians, and scientists seem to have seasonal debates about eggs and their validity in the nutrition realm, particularly in the area of cholesterol, but you can rest easy. In fact, eggs are good for you and can help you stay thin. One study published by the International Journal of Obesity found that participants who had eaten two eggs for breakfast for eight weeks lost 65% more weight than those who ate bagels for breakfast.
While most dieters eat just the egg whites, the yolks contain half the protein of an egg—and a lot of the nutrients. The high protein content is what makes eggs so great. One large egg contains about six grams of protein and nine amino acids with only 77 calories and five grams of fat. That keeps you satisfied for longer and prevents unhealthy snacking. The proteins also stimulate the release of glucagon, which aids in metabolism and can help burn belly fat.
Along with protein, eggs are rich in:
- Vitamins A, B12, and C
- Iron
- Phosphorous
- Selenium
- Almonds
Almonds are high-quality nuts that are rich in vitamin E and monounsaturated fats—the good kind of fat. Almonds are packed with fiber, keeping you full without adding extra calories. They contain magnesium and vitamin B2, which can combat stress, calm nerves, and boost your energy levels. Almonds also contain zinc to curb sugar cravings and oleic oils to curb hunger pangs. If that’s not enough, almonds are also good for your skin and hair.
Almonds also happen to be easy to include in your diet. Snack on a handful at your desk or sprinkle chopped almonds on your meal for added crunch and flavor.
- Chia Seeds
If you’re looking for fiber, you needn’t look any further. Chia seeds are extremely high in soluble fiber. This prevents the absorption of fat, lowers cholesterol, and stabilizes blood sugar levels. The high fiber acts like a sponge, allowing chia seeds to soak up to twenty times their weight in sugar and liquids. As the chia seeds plump up, they expand in your stomach, keeping you full for longer without the extra calories. They are also rich in calcium, iron, and omega-3 fatty acids.
Add chia seeds to your oatmeal or create a healthy pudding of chia seeds and unsweetened chocolate almond milk.
- Quinoa
Although it’s a grain, quinoa acts more like a vegetable and is a close relative of kale, spinach, and Swiss chard. High in fiber and protein, quinoa offers a sturdy foundation for healthy fullness while also offering plenty of vitamins and minerals, including iron and vitamin B12. Quinoa has a low glycemic index, so it won’t cause spikes in blood sugar. One serving of cooked quinoa only has about 172 calories.
The best part about quinoa: you can eat it with just about anything. Eat it plain, add it to veggies, salads, or consume with cinnamon, banana, and almond flakes for an oatmeal replacement. The possibilities with this gluten-free grain are endless.
- Grapefruit
Sweet and tangy, grapefruit is a great source of vitamin C and an antioxidant called lycopene, but these treats may also offer a helping hand in weight loss. One study conducted on mice found that daily grapefruit juice intake expedited weight loss, improved blood glucose, and controlled insulin levels.
Some studies suggest that the mere smell of grapefruit is enough to aid in weight loss, but feel free to go in for the real thing, raw or juiced.
- Yogurt
Yogurt offers a healthy dose of calcium, vitamin D, and protein to keep you full, but probiotics are the key fat-burning ingredient in yogurt. Consuming active bacteria may seem unpleasant, but probiotics are the type of germs that your body needs. They reduce the amount of fat your body absorbs and add flora to your gut that improve your digestion. Eat yogurt plain, add fruit, or use it for creamy sauces. Opt for an unsweetened Greek yogurt instead of regular if you can—Greek yogurt has almost twice the protein and fewer carbs and sodium than regular yogurt.
Food gives you the energy you need to live life to the fullest. Instead of depriving yourself, learn to eat the right things to keep your body healthy and fit. What are some of your favorite foods for weight loss?
Sources:
http://www.fitnessmagazine.com/weight-loss/eating/weight-loss-foods/
http://www.ahealthiermichigan.org/2014/08/23/two-eggs-in-the-morning-can-help-you-lose-weight/
http://metro.co.uk/2013/10/30/how-almonds-can-help-you-to-lose-weight-4163723/
http://www.doctoroz.com/slideshow/5-ways-chia-can-change-your-life?gallery=true
http://www.fitday.com/fitness-articles/fitness/weight-loss/4-reasons-the-quinoa-grain-can-help-you-lose-weight.html#b
http://www.popsugar.com/fitness/Ways-Grapefruit-Can-Help-You-Lose-Weight-26331222
http://www.fitday.com/fitness-articles/fitness/yogurt-smackdown-greek-vs-regular.html#b